AKAP Accreditation Organization

$400.00 – $460.00Price range: $400.00 through $460.00
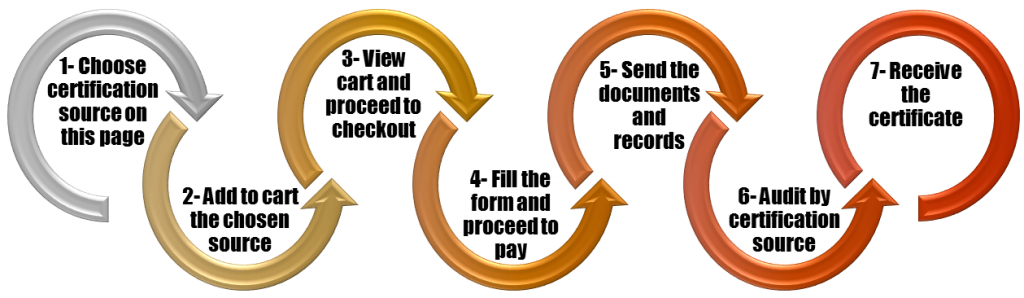
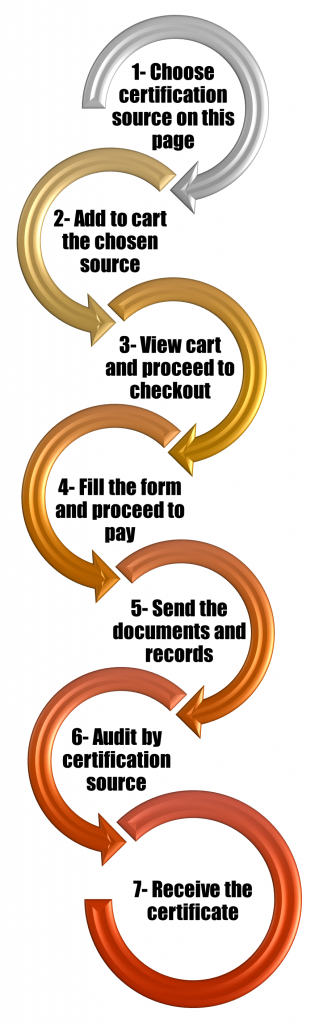
Further details
ISO 15189 (medical laboratories-quality and competence) is developed by the ISO / TC 212 Technical Committee and is used to evaluate clinical laboratories and laboratory diagnostic systems. The purpose of developing ISO 15189 is to determine the specific requirements for quality and qualification in medical laboratories.
ISO 15189 (medical laboratories-quality and competence) is based on ISO / IEC 17025 and ISO 9001. Provides requirements for qualifications and quality specific to medical laboratories. Medical laboratory services are essential in patient care and should therefore be available to meet the needs of all patients and clinical staff who are responsible for patient care.
Such services include arrangements for admission, patient preparation, patient identification, sample collection, transfer, storage, processing, clinical sample testing, and subsequent validation, interpretation, reporting, and consultation, taking into account safety and principles of morals are in the activities of the medical laboratory. So lab technicians tried to come up with a standard that met all the basic needs of a medical diagnostic lab. This standard was named and published as Standard15189.
ISO 15189 (medical laboratories-quality and competence) can be used by medical laboratories in developing their quality management systems and assessing their own competence. It can also be used for confirming or recognizing the competence of medical laboratories by laboratory customers, regulating authorities and accreditation bodies.
What is the standard purpose of ISO15189?
Compliance with the standard helps the laboratory to do its job well, accurately and according to the standards and provides a benchmark for maintaining laboratory skills.
Compliance with the standard is the most effective marketing tool for a medical diagnostic laboratory and can be a passport for contractors who need the approval of their contracting laboratory.
Benefits of ISO implementation:
– Increase customer and consumer confidence
– Increasing the quality of products/services
– Reduce waste and losses in products/services and ultimately reduce costs
– Save on consumables and increase profits
– Planning, implementation of affairs in the form of a predefined international system
– Improve performance, increase productivity and speed in affairs
– Increase efficiency and customer satisfaction
– Reducing the number of complaints
– Improvement and uniformity in the quality of products/services
– Timely delivery of products/services
– Global competition
– Prevent or reduce unexpected events
– Earning points in tenders, obtaining ranks and grades from government organizations, providing evidence in exports
Other benefits are:
– Advertising use in headers, company site and all advertising matters
– Earn points in selecting sample units
– Reduce waste and waste time
– Creating confidence inside and outside the organization
– Transparency of processes and indicators
– Ensuring that customer needs and expectations are met
– Production of product/service with better quality
– Help with more marketing and sales and create demand
– Increasing the productivity and motivation of human resources
– Correcting errors and preventing their recurrence
– Improving communication within the organization
– Prevention of non-compliant product/service production
– Develop sales methods and provide after-sales service
$390.00 – $410.00Price range: $390.00 through $410.00
$410.00 – $440.00Price range: $410.00 through $440.00
$380.00 – $400.00Price range: $380.00 through $400.00
$450.00 – $500.00Price range: $450.00 through $500.00
$370.00 – $400.00Price range: $370.00 through $400.00