AKAP Accreditation Organization
$380.00 – $400.00Price range: $380.00 through $400.00
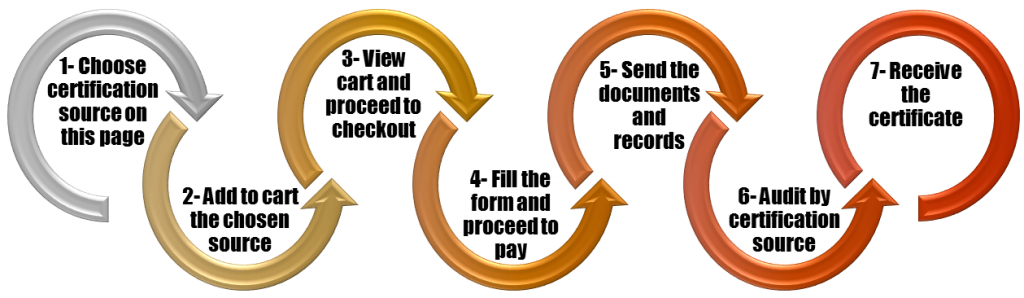
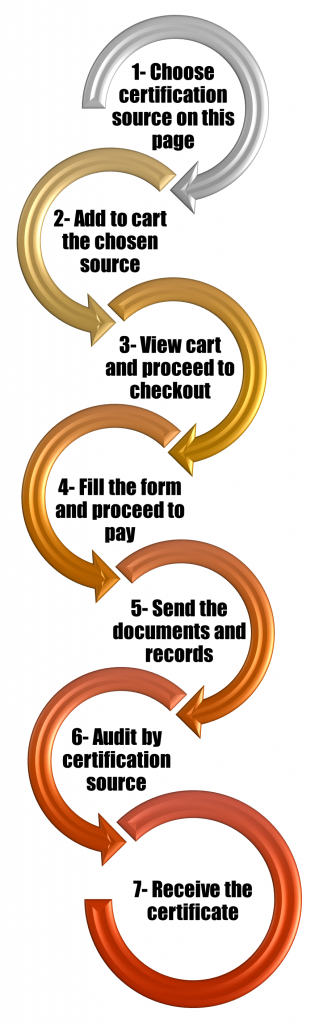
Further details
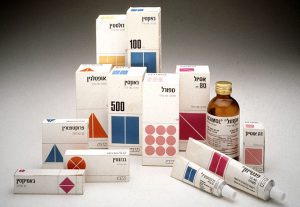
ISO 15378 (primary packaging materials for medicinal products) is an application standard for the design, manufacture and supply of primary packaging materials for medicinal products.
ISO 15378 specifies requirements for a quality management system when an organization:
a) needs to demonstrate its ability to consistently provide products and services that meet customer and applicable statutory and regulatory requirements, and
b) aims to enhance customer satisfaction through the effective application of the system, including processes for improvement of the system and the assurance of conformity to customer and applicable statutory and regulatory requirements.
All the requirements of this International Standard are generic and are intended to be applicable to any organization, regardless of its type or size, or the products and services it provides.
Strict drug requirements also apply to basic packaging materials (those that come in direct contact with the drug). Medicinal products and basic packaging materials should be considered the same. The requirements must be met by the pharmaceutical industry, and of course these requirements are passed on to the manufacturers of the original packaging materials. This creates a very precise process, process stability and quality assurance of the documentary.
ISO 15378 (primary packaging materials for medicinal products) specifies requirements for a quality management system where an organization needs to demonstrate its ability to provide primary packaging materials for medicinal products, which consistently meet customer requirements, including regulatory requirements and International Standards applicable to primary packaging materials.
Benefits of ISO 15378 (primary packaging materials for medicinal products):
-Reduce costs
-Improve communication (between primary packaging manufacturers and the pharmaceutical industry)
-Global standardization of primary packaging materials
-Determine the minimum requirements
-Accepting the international standard as a solution for this industry, therefore, does not require additional legal requirements.
Other benefits of this standard include avoiding qualitative and quantitative degradation, improving the process of environmental protection and creating safety.

Benefits of ISO implementation:
– Increase customer and consumer confidence
– Increasing the quality of products/services
– Reduce waste and losses in products/services and ultimately reduce costs
– Save on consumables and increase profits
– Planning, implementation of affairs in the form of a predefined international system
– Improve performance, increase productivity and speed in affairs
– Increase efficiency and customer satisfaction
– Reducing the number of complaints
– Improvement and uniformity in the quality of products/services
– Timely delivery of products/services
– Global competition
– Prevent or reduce unexpected events
– Earning points in tenders, obtaining ranks and grades from government organizations, providing evidence in exports
– Advertising use in headers, company site and all advertising matters
– Earn points in selecting sample units
– Reduce waste and waste time
– Creating confidence inside and outside the organization
– Transparency of processes and indicators
– Ensuring that customer needs and expectations are met
– Production of product/service with better quality
– Help with more marketing and sales and create demand
– Increasing the productivity and motivation of human resources
– Correcting errors and preventing their recurrence
– Improving communication within the organization
– Prevention of non-compliant product/service production
– Develop sales methods and provide after-sales service
$390.00 – $410.00Price range: $390.00 through $410.00
$410.00 – $440.00Price range: $410.00 through $440.00
$400.00 – $460.00Price range: $400.00 through $460.00
$450.00 – $500.00Price range: $450.00 through $500.00
$370.00 – $400.00Price range: $370.00 through $400.00