AKAP Accreditation Organization

$410.00 – $440.00
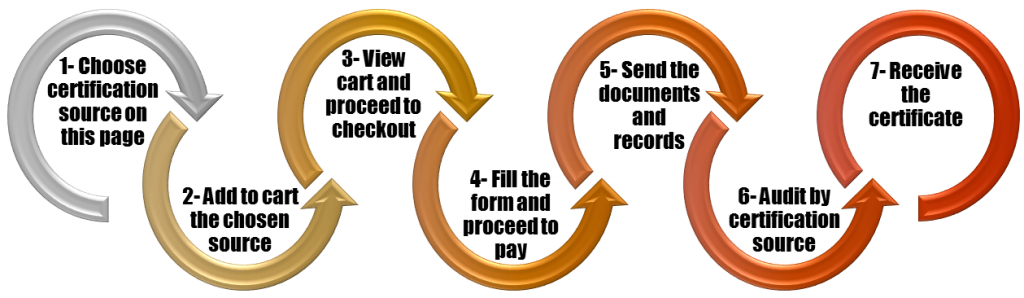
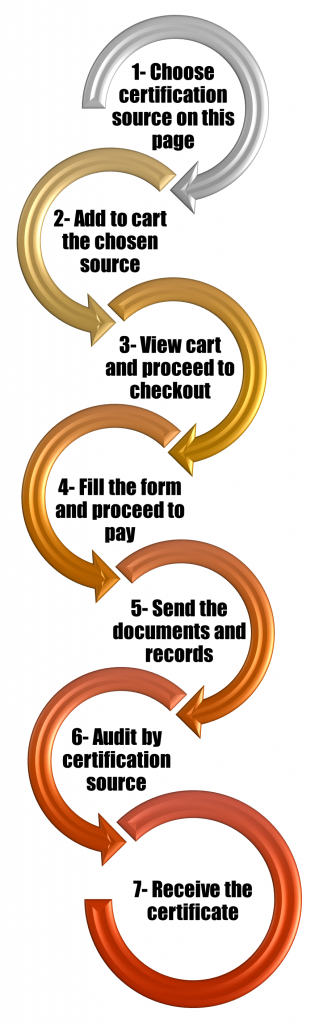
Further details
The ISO/IEC 17025 standard specifies the general requirements for qualifying for tests or calibration as well as sampling.
The term IEC stands for International Electrotechnical Commission which in cooperation with ISO creates the specific system for global standardization. ISO/IEC 17025 is an international standard for testing and calibration laboratories. It was established with the aim of offering quality and improving the processes within laboratories. ISO/IEC 17025 has two key clauses; Management Requirements which are associated with the performance and efficiency of the Quality Management System inside the laboratory, and Technical Requirements which focus on the competencies of employees, testing methodology, equipment, and the test and calibration results.
ISO 17025 Guidelines can be used by all test and calibration organizations. This standard includes, for example, the first, second, and laboratories in which the test is part of the product and inspection certificate.
ISO 17025 is applicable to all organizations performing laboratory activities, regardless of the number of personnel in the organization or the scope of the test or calibration activities.
Laboratory customers, regulatory authorities, organizations and schemes using peer-assessment, accreditation bodies, and others use ISO/IEC 17025 in confirming or recognizing the competence of laboratories.
Becoming certified against ISO 17025 demonstrates your commitment to implement the requirements of this standard. As a certified professional, you will enable laboratories to demonstrate they operate competently, and are able to generate valid results. In addition, you will be able to increase your job opportunities because there are many large laboratory companies which will value your comprehensive knowledge as a professional in this field. As a matter of fact, many organizations have started to offer contracts only to certified professionals and laboratories, as the majority of customers prefer to receive services from certified labs, consequently, enabling you to maximize your earning potential.
Advantages and benefits of ISO 17025 :
-Improve your reputation
-Create new business connections
-Gain competitive advantage
-Increase laboratory effectiveness
-Gain access to more contracts for testing and calibration
-Offer proficiency on improving work processes
-Offer more reliable and efficient lab testing and results
-Achieve customer reliability
-Ensure the accuracy of the tests performed
-Globalization and creating a competitive advantage in exports
-Receive a valid international certificate with a registration code
-Creating a sense of confidence in customers
-Manage and eliminate errors in test laboratories
-Creating and improving an efficient management system
is the main ISO standard used by testing and calibration laboratories. In most countries, ISO/IEC 17025 is the standard for which most labs must hold accreditation in order to be deemed technically competent. In many cases, suppliers and regulatory authorities will not accept test or calibration results from a lab that is not accredited.

Benefits of ISO implementation:
– Increase customer and consumer confidence
– Increasing the quality of products/services
– Reduce waste and losses in products/services and ultimately reduce costs
– Save on consumables and increase profits
– Planning, implementation of affairs in the form of a predefined international system
– Improve performance, increase productivity and speed in affairs
– Increase efficiency and customer satisfaction
– Reducing the number of complaints
– Improvement and uniformity in the quality of products/services
– Timely delivery of products/services
– Global competition
– Prevent or reduce unexpected events
– Earning points in tenders, obtaining ranks and grades from government organizations, providing evidence in exports
– Advertising use in headers, company site and all advertising matters
– Earn points in selecting sample units
– Reduce waste and waste time
– Creating confidence inside and outside the organization
– Transparency of processes and indicators
– Ensuring that customer needs and expectations are met
– Production of product/service with better quality
– Help with more marketing and sales and create demand
– Increasing the productivity and motivation of human resources
– Correcting errors and preventing their recurrence
– Improving communication within the organization
– Prevention of non-compliant product/service production
– Develop sales methods and provide after-sales service
$390.00 – $410.00
$380.00 – $400.00