AKAP Accreditation Organization

$390.00 – $410.00
Further details
The IWA 1 standard helps all medical centers to plan and implement the terms and conditions of these medical centers using ISO 9001 quality management system.
IWA 1 Application Scope:
All organizations related to health care services that work in the field of management and provision of health care services and products, including educational and research centers, hospitals, offices and monitoring centers and other related organizations regardless of type, size, product and service that Provide, and manufacturers and distributors of pharmaceutical products, due to the importance of processes and services in health centers, the requirements of the quality management system in these centers are interpreted differently than other organizations.
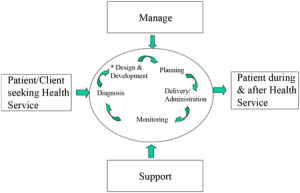
IWA 1 provides additional guidance for any health service organization involved in the management, delivery, or administration of health service products or services, including training and/or research, in the life continuum process for human beings, regardless of type, size and the product or service provided.
The definitions of terms such as patient/client, client, primary, ancillary, and specialty care vary by region within the health service community. The organization’s processes for addressing these activities should be included in the quality management system. The recommendations and guidance in this document apply to anyone in the organization who could affect the quality of the organization’s product(s) or service(s), including necessary support services.
IWA1 provides guidelines beyond the requirements given in ISO 9001 in order to consider both the effectiveness and efficiency of a quality management system, and consequently the potential for improvement of the performance of an organization. When compared to ISO 9001, the objectives of customer satisfaction and product quality are extended to include the satisfaction of interested parties and the performance of the organization.
IWA 1 is applicable to the processes of the organization and consequently the quality management principles on which it is based can be deployed throughout the organization. The focus of this International Standard is the achievement of ongoing improvement, measured through the satisfaction of customers and other interested parties.
Applicant organizations:
-Hospitals and medical centers, including public, private and medical charities
-Medical complexes / treatment clinics
-Medical and dental offices (surgical and non-surgical)
-Emergency centers and services for patients and the disabled
-Patient transportation centers
-Psychiatric rehabilitation centers
-Rehabilitation centers for patients addicted to drugs and psychotropic substances
-Medical laboratories (laboratories can establish this standard as the basic laboratory standard or the ISO 15189 standard
-Centers for the Disabled and Physical Rehabilitation
The purposes of developing the IWA 1 standard for health centers:
-Improving the quality and safety of health care services
-Improve processes to create added value for the organization and the customer
-Improving the organization’s image in the community
-Increase customer trust and access to tools to assess quality with a specialized approach
Advantages of implementing quality management system with IWA1 approach
-Improve service quality
-Increase employee ability and improve the work environment
-Reduce costs due to low quality
-Ensuring compliance with legal requirements
Benefits of ISO implementation:
– Increase customer and consumer confidence
– Increasing the quality of products/services
– Reduce waste and losses in products/services and ultimately reduce costs
– Save on consumables and increase profits
– Planning, implementation of affairs in the form of a predefined international system
– Improve performance, increase productivity and speed in affairs
– Increase efficiency and customer satisfaction
– Reducing the number of complaints
– Improvement and uniformity in the quality of products/services
– Timely delivery of products/services
– Global competition
– Prevent or reduce unexpected events
– Earning points in tenders, obtaining ranks and grades from government organizations, providing evidence in exports
– Advertising use in headers, company site and all advertising matters
– Earn points in selecting sample units
– Reduce waste and waste time
– Creating confidence inside and outside the organization
– Transparency of processes and indicators
– Ensuring that customer needs and expectations are met
– Production of product/service with better quality
– Help with more marketing and sales and create demand
– Increasing the productivity and motivation of human resources
– Correcting errors and preventing their recurrence
– Improving communication within the organization
– Prevention of non-compliant product/service production
– Develop sales methods and provide after-sales service
$410.00 – $440.00
$380.00 – $400.00